In these difficult days we are in, another worrying development occurred on April 4, 2020. A forest fire that started near Chernobyl alarmed the whole world.
While it is stated that someone caused the fire by burning dry grass near a prohibited area, officials said they face similar situations every year. So what are the environmental risks of this fire? We will address this in our article, but first, I would like to briefly touch on nuclear energy and nuclear power plants for better understanding.

Nuclear Energy has continuously occupied the world's agenda from the past to the present due to humanity's need for energy in every field and has led to many discussions. Nuclear Power Plants, which first entered our lives in the 1950s, continue to operate today. As of March 2020, 10% of the world's electricity is still provided by 440 operational Nuclear Power Plants. Additionally, 50 Nuclear Power Plants are under construction.
We can roughly outline the development of Nuclear Technology as follows;
Between 1895 and 1945, emphasis was placed on Atomic Radiation, Nuclear Fission, and the Science of Atomic Change. Most of the developments occurred in the last six years of this period.
Between 1939 and 1945, intensive work was carried out on the Atomic Bomb.
From 1945 onwards, attention focused on the controlled use of this energy for military naval deployment and electricity generation.
Since 1956, reliable and sustainable nuclear power plant technologies for commercial purposes have become the focal point.
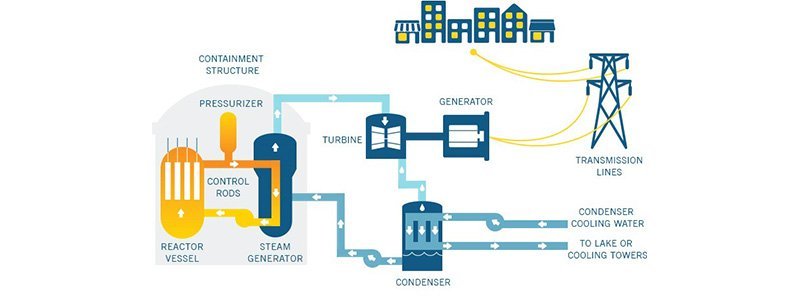
How Do Nuclear Power Plants Work? Let's Take a Look at Their Operation!

Nuclear power plants are very similar to fossil fuel power plants in terms of process. Both types of plants require a cooling system and a turbine that will be driven by steam obtained from it. The difference is that the energy source in nuclear power plants is nuclear reactions.
The heat generated inside the reactor vessel is transferred to the coolant. As a coolant, water is used in existing commercial nuclear plants.is split into two smaller atomic nuclei. When the fission reaction is initiated, it continues in a chain reaction.When a 235U atomic nucleus captures a moving neutron, it splits and releases heat, during which 2 or 3 neutrons are also emitted.It starts to fizzle and the reaction continues in a chain reaction. The uranium used in commercial reactors has been enriched compared to its natural state. This way, it contains more 235U isotopes than the normal amount. To achieve this, it must go through a series of enrichment processes. To avoid making our article too lengthy, it can be roughly stated that the concentration of 235U in the prepared fuels is increased from 0.7% to a range of 2%-5%. There are also commercial reactors that use natural uranium as fuel. The 238U isotope, which is the part of the fuel excluding 235U, can only undergo fission as a result of collisions with neutrons at certain energy levels, but most of these collisions usually result in the neutron being absorbed and the transformation of 238U into Plutonium-239.This isotope of plutonium can undergo fission as a result of collisions with thermal or fast neutrons. In water reactors, its contribution to normal energy production gradually increases until it reaches 30% of the power generated.**What Are Nuclear Wastes? What Wastes Are Generated as a Result of Processes and How Are They Disposed Of?**We are slowly reaching the part of our article that we will address. The part that concerns us is the isotopes that are significant in terms of the radioactivity that will be generated as a result of this reaction. Important fission products that will emerge from the fission of 235U, in terms of probability and radioactivity, are Bromine (Br), Cesium (Cs), Iodine (I), Krypton (Kr), Strontium (Sr), and Xenon (Xe). Like any radioactive element, these isotopes also undergo decay over periods referred to as half-lives, which are measured in different durations. Due to their quantities and radioactivities, these isotopes and decay products constitute a significant part of nuclear waste.For example, the radioactive isotope of iodine, 129I, has a half-life of 16 million years. In this regard, the products released as a result of fission pose a significant environmental threat.The uranium used in the reactor is found in the form of ceramic pellets inside metal rods.These metal fuel rods must be disposed of after the reaction is complete. However, a series of chemical processes are applied to recover the remaining unused uranium, resulting in HLW (High-Level Waste). Later, these metal rods are temporarily stored in a water-filled pool near the reactor. During this time, decisions are made regarding final storage sites.These liquid wastes are vitrified, placed in steel containers, and then buried deep within the earth in a tomb. The areas where they are buried are determined by the state.Intermediate-level wastes (ILW), which can be characterized as reactor waste and chemicals, are solidified in concrete or tar and buried.**So, What Risk Did the Chernobyl Forest Fire Pose? Let's Take a Look at a Fire That May Seem Like a Normal Forest Fire but Is Not Quite So.**On Saturday, April 26, 1984, a massive explosion occurred in Reactor No. 4 of the Chernobyl Nuclear Power Plant, releasing a very high amount of radiation into the surrounding area. The radiation lasted for days, and particles spread over kilometers. Cloud clusters reached Europe and Turkey via air currents over the Black Sea. The Chernobyl disaster, classified as level 7 on the International Nuclear Event Scale, turned the city of Pripyat and the town of Chernobyl into ghost towns. Nature began to reclaim the area, and the existing forests expanded significantly, asserting dominance in the region.Plants in their environment can absorb and store many elements from the air, soil, and water. This also applies to isotopes that spread and cause contamination.As you might expect, the plants currently found in the area contain high amounts of radioactive isotopes, and these isotopes can contaminate the soil even if they turn to ash, dissolve in water, and persist in the environment.For example, the Cesium-137 isotopereacts with water to form cesium hydroxide, a soluble compound. Cesium-137 is a chemically very active isotope and its management is difficult. When cesium-137 is taken into the body in soluble form, it distributes evenly throughout all soft tissue and can lead to death within an average of 30 days.During the fire, the measured values of Cesium-137 showed a 16-fold increase. The reason for this is that the fire released radioactive particles from the biomass, allowing them to mix with the air or enrich as ash. If the fire had reached the Chernobyl Plant, not only nuclear fuel residues but also many fission products such as isotopes of cesium, iodine, and strontium would have been released into the atmosphere, threatening human health as they spread through air currents.For now, the statements made by Ukrainian officials indicate that the measured values near Chernobyl, in Ukraine's capital Kyiv, are normal. Although it is said that momentary increases were observed around the burning area during the fire, the radioactive particles carried by the smoke will certainly affect the nearby environment through rain.We hope that humanity does not have to pay heavy prices for similar events. The world is ours, control is in our hands; it is our sole duty to protect our environment and to pass on resources that can sustain life to future generations. **Sources:**Sumio Itoh, Tetsuya Eguchi, Naoto Kato & Shigeru Takahashi (2014) Radioactive particles in soil, plant, and dust samples after the Fukushima nuclear accident, Soil Science and Plant Nutrition
B.D.Amiro S.C.Sheppard F.L.Johnston W.G.Evenden D.R.Harris, Burning radionuclide question: What happens to iodine, cesium and chlorine in biomass fires?, Science of The Total Environment Volume 187, Issue 2, 30 August 1996, Pages 93-103
Dr.Stephen G. Pallardy, Physiology of Woody Plants (Third Edition), 2008
https://www.world-nuclear.org/information-library/nuclear-fuel-cycle/nuclear-wastes/radioactive-waste-management.aspx
https://www.world-nuclear.org/information-library/nuclear-fuel-cycle/nuclear-power-reactors/nuclear-power-reactors.aspx
https://www.taek.gov.tr
https://www.sciencealert.com/fires-in-chernobyl-exclusion-zone-have-now-reached-the-ghost-town-of-pripyat
“Environmental consequences of the Chernobyl accident and their remediation: Twenty years of experience. Report of the Chernobyl Forum Expert Group ‘Environment’,” (PDF)(English). Vienna: International Atomic Energy Agency.